This post has been contributed by Prof Suma Athreye, University of Essex and CIMR Fellow
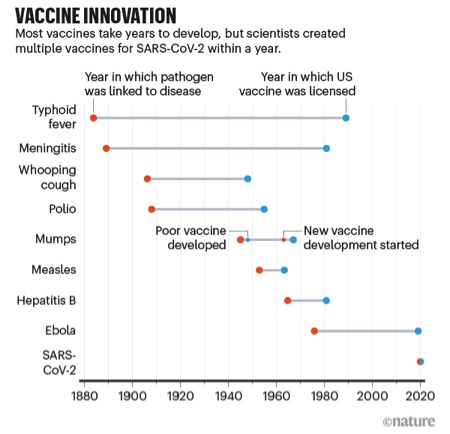
Vaccines to combat the threat of Covid-19 have been developed at an amazing speed – in under a year. The figure below, from the journal Nature, puts this spectacular success in perspective — the fastest any vaccine had previously been developed, from viral sampling to approval, was four years, for mumps in the 1960s. And while the first vaccines to be approved came from the private sector using new mRNA technologies, the unsung heroes of the COVD-19 vaccine effort have been the publicly funded national universities and laboratories who were able to assemble vaccines on no less than 10 different vaccine platforms based on decades of research and scholarship.
Evidence that University and public sector research have risen to the occasion in the development of a vaccine to contain the COVID-19 pandemic comes from the candidate vaccines in clinical trials maintained by the WHO (based on last access on 15 February 2021). 2 of the 3 UK-approved vaccines (Moderna and Oxford Astra Zeneca) and 8 of 16 vaccines in phase 3 trials have university and public sector links, while this number rises to 29 when we consider all the 63 candidate vaccines in clinical trials. These numbers represent a much higher percentage than the 20% normally reported as collaborative innovation involving firms and universities in Innovation Surveys. Undoubtedly, decades of investment in public sector research delivered because national governments, multilateral organisations like CEPI and philanthropists like Bill Gates gave large sums of money to enable necessary clinical trials. Neither money nor good science could have achieved this outcome alone– but their combination defied the large odds that are common in vaccine development.
In several ways, the development of the COVID-19 vaccine parallels the inter-war period search for antibiotics described by Bhaven Sampat in his work. Bacterial disease had been the main killer of soldiers in the First World War and earlier wars and so governments of all the major countries heading towards a Second World War had sponsored antibiotic research using public funds. The discovery of antibiotics proceeded along three main research platforms – sulpha drugs (developed in Germany by Gerhardt Domgk in the 1930s), penicillin (developed in the United Kingdom in the 1930 by Alexander Fleming, and by Howard Florey and Ernest Chain at Oxford, but first mass produced in the US later), and streptomycin (developed by Wakesman in the US in the 1940s). Many second generation antibiotics were follow on innovations along these three platforms– including semi-synthetic penicillins, cephalosporins, and a range of broad spectrum antibiotics. The eerie parallels (Germany, Oxford and the US as the sites of the first discoveries), are numerous but in this blog I want to focus attention on what contributed to successful knowledge transfer from the university science base to industry.
First, in both the discovery of antibiotics and the COVID vaccine, there was a huge source of demand for innovation that was exogenous – as Nathan Rosenberg among others noted, war (and now the pandemic) focussed scientific minds on a single problem and incentivised solution that would benefit humanity at large. In both cases, the biological science underlying the solution(s) had been known for a while, but a race emerged along a few platforms. In the case of antibiotics there were three main candidates (noted earlier) while the race for a COVID vaccine has emerged with about five dominant vaccine platforms (mRNA, protein subunit, non-replicating viral vector, DNA and inactivated virus). As with antibiotics, the success of some of these platforms will set the basis for future developments in medicine. Thus, the mRNA Covid vaccines are widely expected to pave the way for a more general vaccine (targeting all corona infections for example) and explore the potential for a new drug industry based on personalised medicine.
Second, then as now, new countries are staking their claim to this emerging industry. Although media attention is focussed today on the successes and failures of US and European companies, the vaccine producers for poorer countries may well come from outside this group. Similar to the involvement of US in the development of antibiotics, we see Indian firms leading in the manufacture of vaccines for researchers in the West. Chinese Research labs and universities are involved in clinical trials on 4 of the 5 leading Coronavirus vaccine platforms. China and Russia have also led the effort to supply vaccines to the poorer world. Some estimates suggest that the 424.3 million doses of SinoVac have been supplied by China to 27 countries which include Philippines, Indonesia, Turkey, Egypt, Brazil, Chile and Venezuela. Similarly 388.1 million doses of Sputnik V have been distributed to 20 countries including India, Uzbekistan, Hungary, Mexico and Argentina.
Third, then as now, the university scientists involved worried about the effects of patenting the final products. In Oxford, patenting penicillin was a source of discord between Chain (who wished to patent) and Florey (who didn’t) and a source of much distress for all UK medical researchers when their collaborators in the US Ministry of Agriculture did patent. Apart from the fact that their joint efforts were not acknowledged, UK firms were forced to pay royalty on the use of the patent! Domagk (at Bayer) was held back by the process patent regime in Germany and the fear of reverse engineering by French pharmaceutical manufacturers. Today, product patents are allowed on all drugs and patenting of public science is better accepted thanks to the legacy of the Bayh-Dole Act, but the different priorities of pubic science and commercialisation continue to create ethical dilemmas.
In the UK, the Oxford –Astra Zeneca vaccine has prompted a debate about ethics of privatising profits while socialising the risks of R&D , while the decision to grant an exclusive license to Astra Zeneca for vaccine production appears to be contrary to what the scientists working on the vaccine originally wished. Nevertheless, the ability to patent has given Oxford University some bargaining rights about where the vaccine would go and at what price. And surely the availability of a vaccine that is only as expensive as a cup of coffee is likely to be a game changer and offers hope for poorer countries that do not have the scientific capability to develop their own vaccine for COVID. The transparency of the clinical trial data and their scrutiny by peer review has been a notable feature of public science involvement in both the UK and Russia. Clinical trial data has been less transparent for the other vaccine candidates and this may have contributed to vaccine misinformation.
A last set of parallels are in the delivery of medicines formulated in Universities and Public sector labs to those who need them. Universities needed companies to manufacture the antibiotics and scale up their production. This is not straightforward as the stability of chemical production may not always stay the same with scaling up. The US and UK governments supported the mass production of natural penicillin before World War II and many national governments intervened directly to ensure commercialisation in the public interest. Today, university-industry relations are more institutionalised while governments are active in procurement. The lack of public intermediation in production may have skewed some choices and prompted experimentation. Imperial College also engaged in clinical trials for a potential vaccine have announced a different delivery mechanism. Together with funding from a venture capital fund, they have set up a social enterprise that will deliver vaccines at cost. When we consider vaccine delivery for poorer countries, international organisations like WHO’s COVAX, CEPI and the GAVI alliance may play a larger role—perhaps taking over the role that national governments played in the inter-war period.
When the dust has settled, the COVID vaccine may turn out to be the finest hour for universities who have embraced the third mission of socially useful research. In the last three decades, developed and developing countries have implemented strategies to support the application or commercialization of knowledge produced by public research organizations. However, the difficulties of COVID vaccine delivery underline the long way we still need to go to evolve a successful delivery mechanism of public research for maximum social value.
How can policymakers improve the efficiency of knowledge transfer practices to help seek practical solutions to other critical societal challenges such as tropical disease and climate change? Can Emerging Markets emerge as important sources of public science based solutions? Harnessing Public Research for Innovation in the 21st Century, aims to develop a conceptual framework to evaluate knowledge transfer practices and outcomes, improve knowledge transfer metrics, surveys and evaluation frameworks, generate findings on what does and does not work, and propose related policy lessons. CIMR colleagues Federica Rossi and Suma Athreye have contributed their research which studies knowledge transfer from the science base in the UK and draws policy lessons for the different incentives required for knowledge transfer in high and middle income countries.
“A shorter version of this blog post was published on the Cambridge University Press blog on 4 March 2021”

